An absolutely perfectly pure, "white" diamond would be composed of carbon atoms arranged in a perfect tetrahedral lattice. With this atomic structure, none of the light would be absorbed, the diamond would return all the light it receives and would be perceived as completely colorless.
However such diamonds don't exist: during their crystallization phase, diamonds lie 150 to 200 km under the earth's crust, and are subjected to pressures of about 70,000 kg/cm2 (about 1,000,000 pounds/in2) and to temperatures ranging from 1300 to 2000°C. (2300 to 3600°F).
Under these conditions, diamonds lose their atomic integrity: their orderly tetrahedral structure is twisted and they come to absorb other types of atoms. As a result, they do not reflect all the light they receive - some of the wavelengths are absorbed and the diamond takes on some color.
Diamond Classification
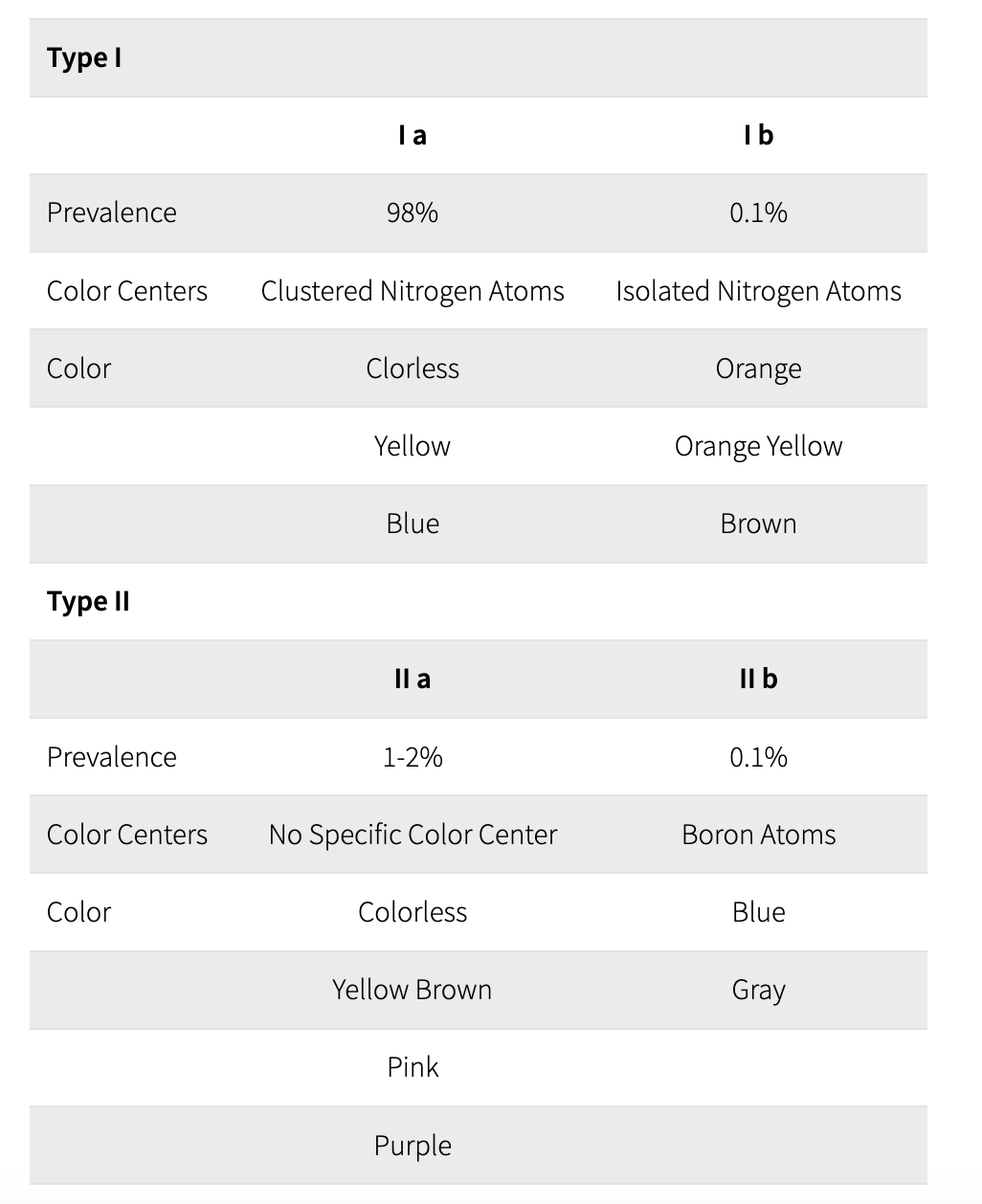
The color of diamonds depends on their chemical composition. The atoms contained in the stones will determine their reaction to light, thus changing their color. Diamonds are classified following what happened to their structure during the crystallization process. They are divided into two groups: type I and type II, the second one being by far the rarest.
Type I Diamonds
Are diamonds that ended up absorbing a detectable quantity of nitrogen atoms, which absorb blue light.

Type Ia: If the nitrogen atoms are clustered together within the carbon lattice, then the diamond is said to be a Type Ia diamond. Because these diamonds absorb blue light, they can have a pale yellow color. 98% of diamonds are Type Ia. However, scientists have recently discovered that blue diamonds from the Argyle mine in Australia could belong to Type Ia.
Type Ib: If the nitrogen atoms are evenly spread out throughout the carbon lattice, then the diamond is said to be a Type Ib diamond. These diamonds absorb green light as well as blue light, and have a darker color than type Ia diamonds. Depending on the precise concentration and spread of the nitrogen atoms, these diamonds can appear deep yellow ("canary"), orange, brown, greenish. Less than 0.1% of diamonds belong to Type Ib.
Type II Diamonds
Are diamonds that absorb no, or very few, nitrogen atoms.

Type IIa: These diamonds can be considered as the "purest of the pure" - they contain no, or minuscule amounts of other elements and are usually colorless. Unless, that is, the carbon tetrahedrons that make up the diamond were twisted and bent out of shape while the diamond rose to the surface of the earth.An imperfect carbon lattice will make the diamond absorb some light, which will give it a yellow, brown or even pink or red color. 1-2% of diamonds belong to Type IIa.
Type IIb:These diamonds contain no nitrogen - but they contain boron, which absorbs red, orange and yellow light. These diamonds therefore usually appear to be blue, although they can also be grey or nearly colorless. A part of naturally blue diamonds belong to Type IIb, which makes up 0.1% of all diamonds. Blue diamonds from South Africa belong to that type.
Summary of Diamond Classification
What About Green Diamonds?
Green diamonds are a separate case: these diamonds can contain clustered nitrogen atoms or they can contain no nitrogen atoms - what gives them their color is that they have been bombarded by nuclear rays during their growth. Of course, these are harmless. Interestingly, this bombardment makes them absorb magenta wavelengths, which gives them their green color. These diamonds are extremely rare.












